What happens if two atoms of equal electronegativity bond together?
If the atoms are equally electronegative, both have the same tendency to attract the bonding pair of electrons, and so it will be found on average half way between the two atoms. To get a bond like this, A and B would usually have to be the same atom. You will find this sort of bond in, for example, H2 or Cl2 molecules.
This sort of bond could be thought of as being a "pure" covalent bond - where the electrons are shared evenly between the two atoms.
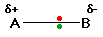
What happens if B is slightly more electronegative than A?
That means that:
The B end of the bond has more than its fair share of electron density and so becomes slightly negative.
At the same time, the A end (rather short of electrons) becomes slightly positive.
Important Notes:
Electro negativity is the average of ionization potential and the electron affinity
Electro negativity refers to atom in the molecular state
Electron affinity refers to atom in single state
In horizontal period: Electro negativity increases from left to right due to:
Decreasing of atomic radius.
Increasing in positively nuclear charge that increase the ability of the atom to attract the electrons of chemical bondIn vertical group: Electro negativity decreases from up to down due to:
Increasing of atomic radius.
Increasing of extra energy levels.
Decreasing of the ability of the atom to attract the electrons of chemical bondNotes:
The most electronegative element is fluorine. If you remember that fact, everything becomes easy, because electronegativity must always increase towards fluorine in the Periodic Table.
As you go across a period the electronegativity increases. The chart shows electronegativities from sodium to chlorine - you have to ignore argon. It doesn't have an electronegativity, because it doesn't form bonds.
As you go down a group, electronegativity decreases. (If it increases up to fluorine, it must decrease as you go down.) The chart shows the patterns of electronegativity in Groups 1 and 7.
Related Questions HERE




0 comments:
Post a Comment