Electron Affinity is the energy given off when a neutral atom in the gas phase gains an extra electron to form a negatively charged ion.
In horizontal period:
Electron affinity increases from left to right across the period because of
Increase in nuclear charge and
Decrease in atomic size.
This causes the incoming electron to experience a greater pull of the nucleus thus giving a higher electron affinityIn vertical group:
Electron affinity decreases down the group because
The number of shells increases i.e., the atomic size increases and
The effective nuclear charge decreases.
This causes the incoming electron not to experience much attraction of the nucleus thus giving a lower electron affinityImportant Notes:
The highest affinities are displayed by the elements F and Cl (from the Halogens).
Nobel gases have a higher electron affinity (The electron affinity of completely or half completely filled atoms is almost zero)
Electron Affinity of 9F is lower than that of 17Cl
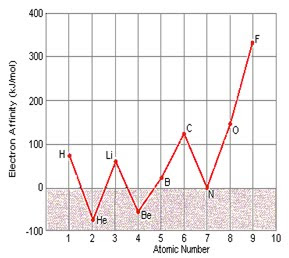
Electron Affinity of 4Be is lower than that of 3Li
Electron Affinity of 7N is lower than that of 6C
Electron Affinity of 12Mg is lower than that of 11Na
Electron Affinity of 15P is lower than that of 16SiExplanation:
In case of Nobel gases: Nobel gases have a completely filled outer shell and any extra electron added would have to go onto the next higher shell which is higher in energy.
Electron Affinity of 9F is lower than that of 17Cl:
9F (2, 7) small radius, more attraction (lower Affinity)
17Cl (2, 8, 7) large radius, less attraction (higher Affinity)
Because of the small size of 9F atom, so any extra electron added would suffer a strong attraction force.
Electron Affinity of 4Be is lower than that of 3Li:
3Li (1s2, 2s1) the outer sublevel P is empty but s is half completely filled
4Be (1s2, 2s2) the sublevel p is empty but s is completely filled
The atom becomes more stable if the outer sublevel is completely filled, half filled, or empty and the adding extra electron decreases the stability of the atom
Electron Affinity of 7N is lower than that of 6C:
6C (1s2, 2s1, 2p2) the outer sublevel P is filled with 2 e-
7N (1s2, 2s2, 2p3) the sublevel p is half completely filled
The atom becomes more stable if the outer sublevel is completely filled, half filled, or empty and the adding extra electron decreases the stability of the atom
The new electron make the C atom more stable because its outer sub shell becomes half completely filled
Electron Affinity of 12Mg is lower than that of 11Na
Electron Affinity of 15P is lower than that of 16Si
Give reason:
Electron Affinity increases from left to right
Electron Affinity decreases from up to down
Nobel gases have a higher electron affinity
Electron Affinity of 9F is lower than that of 17Cl
Electron Affinity of 4Be is lower than that of 3Li
Electron Affinity of 7N is lower than that of 6C
Electron Affinity of 12Mg is lower than that of 11Na
Electron Affinity of 15P is lower than that of 16Si
Electron Affinity values for beryllium, nitrogen, and neon disagree with the rest of period 2
4Be (1s2, 2s2) the outer sublevel p is empty
7N (1s2, 2s2, 2p3) the outer sublevel p is half filled
10Ne (1s2, 2s2, 2p6) the outer sublevel p is completely filled
The atom becomes more stable if the outer sublevel is completely filled, half filled, or empty and the adding extra electron decreases the stability of the atom.





0 comments:
Post a Comment